


The Science
Unraveling the Power of Mycoton: Nature, Research, and Transformation
How Mycoton Works
Mycoton is a powerful dietary supplement packed with biologically active ingredients derived from chitin, glucans and melanins. This unique formula is designed to support and restore your health, offering a range of benefits. Whether you want to proactively maintain your well-being or enhance your healing process, Mycoton is your trusted companion. Its effectiveness lies in its natural mechanisms of action, which include:
- Powerful Biosorbent for Gentle Detoxification: Toxin Elimination Technology
Mycoton is renowned as one of the most potent biosorbents available. It effectively eliminates a wide range of toxins that enter our bodies through water, food, inhalation, and even through the skin. This exceptional biosorbent targets heavy metals, radionuclides, and metabolic toxins that accumulate in our system, ensuring a comprehensive detoxification process.
The mechanism of action of Mycoton is ingeniously simple. As depicted in the diagram, toxins find their way into our bodies through various pathways, whether it be through ingestion, inhalation, or natural metabolic processes. Mycoton acts as a powerful enterosorbent that actively absorbs toxins that passively diffuse from circulation into the gastrointestinal tract, trapping and removing these harmful substances from our system.
The human body is a remarkable system with multiple organs dedicated to eliminating toxins and maintaining overall well-being. Key players in this process include the liver, kidneys, gastrointestinal tract, lungs, and skin. These organs work tirelessly, with the bloodstream flowing through them, purging toxins and ensuring our bodies remain clean and healthy. However, when the excretory system encounters disruptions, toxins can accumulate, leading to various diseases and imbalances.
In today's world, our bodies face an onslaught of synthetic toxins from sources like food, water, and medications. These harmful substances can overwhelm even healthy excretory organs, making it challenging for them to effectively remove toxins. To maintain optimal health in such circumstances, additional support is necessary to facilitate the removal of toxins from the bloodstream.
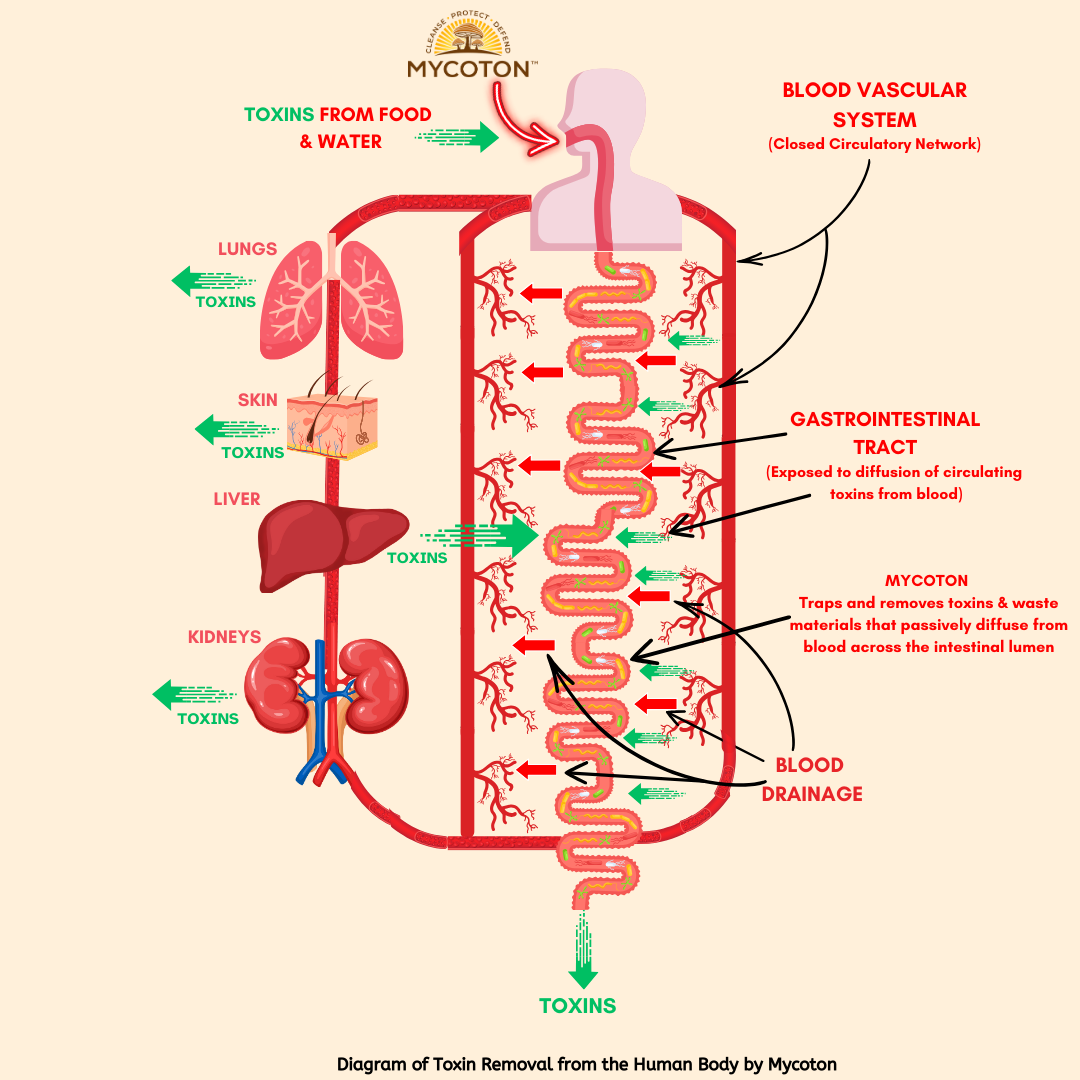
Within the body, most organ systems operate within a closed circulatory network, except for the gastrointestinal tract. This unique pathway allows approximately 10 liters of fluid, including saliva, gastric juice, and digestive byproducts, to enter the gastrointestinal tract daily. Remarkably, this fluid is reabsorbed back into the bloodstream. By strategically placing an adsorbent along this pathway, specifically in the gastrointestinal tract, we can effectively purify the body by trapping and removing different waste materials via passive diffusion across the intestinal lumen.
This is where Mycoton excels. As a highly effective biosorbent, Mycoton fulfills this role with precision. It possesses a wide but selective detoxifying effect, adept at absorbing toxins and heavy metals from the body. Importantly, Mycoton achieves this without disturbing the absorption of essential biogenic trace elements or disrupting mineral metabolism that is crucial for your overall well-being.
Mycoton is a safe and non-toxic solution, free from contraindications and side effects. It has received approval as a food supplement from the Ministry of Public Health Services of Ukraine, further attesting to its quality and reliability.
- Potent Immune Modulation
Mycoton, the remarkable natural supplement, harnesses the power of glucans to exhibit potent immunomodulating properties.
Extensive research into Mycoton's immunomodulating capabilities has been conducted under clinical conditions, specifically on patients with chronic viral hepatitis. These patients received 0.5 grams of Mycoton three times a day as an adjunct to their primary treatment, while a control group underwent treatment without Mycoton. The results were striking.
Mycoton effectively regulates the levels of crucial immunocompetent cells, such as T- and B-lymphocytes, while also reducing immunoglobulin levels. This harmonization of immune parameters plays a pivotal role in promoting overall immune system balance and optimal functionality. Moreover, Mycoton significantly decreases the presence of circulating immune complexes, enhancing your body's immune response. Additionally, it has been observed to greatly improve the function of phagocytosis, the process by which immune cells engulf and eliminate harmful substances.
By embracing Mycoton as part of your wellness journey, you empower your immune system to perform at its best. Whether you are facing existing health concerns or striving to maintain your well-being, Mycoton's immune modulating properties provide targeted support to bolster your body's defenses and promote a state of vibrant health.
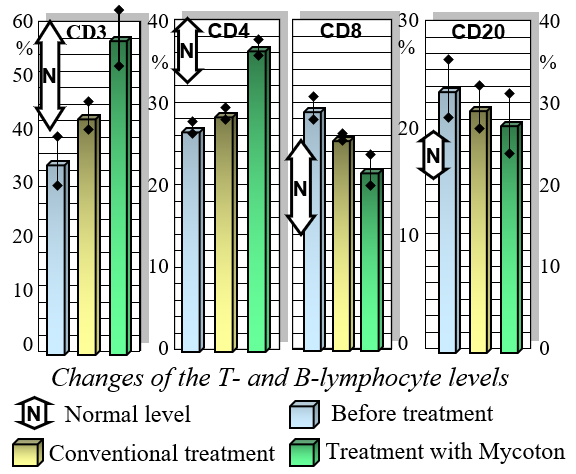
Changes of T-and-B Lymphocytes
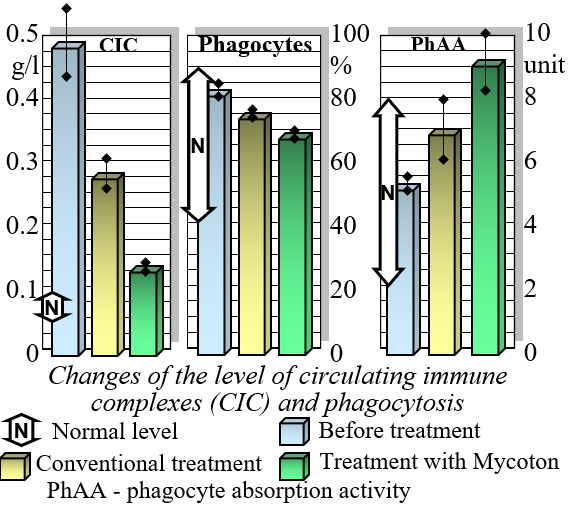
Changes in levels of Circulating Immune Complexes
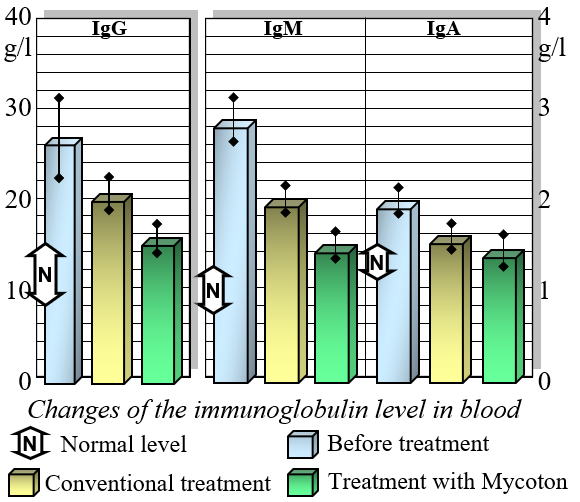
Changes of the Immune Globulin Levels in Blood
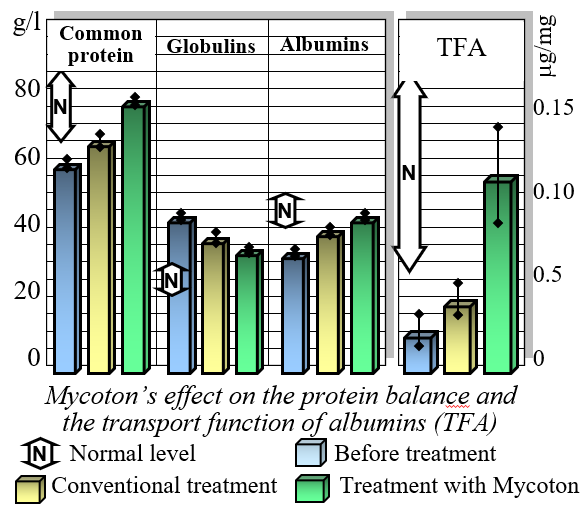
Effect on Protein Balance
- Optimization of Biochemical Homeostasis
In addition to its direct impact on immunocompetent cells, Mycoton possesses a remarkable ability to promote detoxification within the body, thereby exerting a profound influence on the immune system. The application of Mycoton offers a multitude of favorable effects on crucial biochemical parameters that contribute to your overall health.
One of the key benefits of Mycoton is its ability to restore balance to protein levels in the bloodstream. By normalizing the protein composition in the blood plasma, Mycoton reduces the levels of globulins while increasing the content of albumins. Of particular significance is its unique capacity to restore the transport function of albumins. This ensures the efficient delivery of essential nutrients and compounds throughout your body, optimizing cellular functioning and promoting overall well-being.
Furthermore, Mycoton demonstrates its efficacy by promoting the normalization of enzymatic activity. By fostering a harmonious enzymatic environment, Mycoton supports vital metabolic processes and ensures that your body's biochemical reactions function optimally.
In the quest for total wellness, Mycoton plays a pivotal role in regulating cholesterol and bilirubin levels. By normalizing cholesterol levels and addressing bilirubin imbalances, Mycoton supports a healthy cardiovascular system and contributes to the overall balance of biochemical parameters within your body.
Unlock the transformative power of Mycoton and experience the profound benefits of biochemical equilibrium. Embrace this remarkable supplement as an integral part of your wellness journey, and witness the positive impact it can have on your overall health and vitality.
- Pioneering Radioprotection: The Chernobyl Study Unravels Mycoton's Potential
In a groundbreaking study conducted on exposed personell at a shelter inside the Chernobyl nuclear disaster site, researchers made a groundbreaking discovery about Mycoton's radioprotective prowess. The study revealed that Mycoton's Chitin-Melanin-Glucan complex possesses potent antioxidant and immune modulating properties, crucial for shielding the body in radiation-intensive environments. As an adaptogen, Mycoton exhibited the exceptional ability to fortify the human body against escalated radiation exposure, rendering it a trusted and invaluable ally in safeguarding well-being amidst challenging conditions.
- Transforming Gastrointestinal Wellness: Mycoton's Breakthrough in Managing Chronic Lesions of the Digestive Tract
Chronic lesions of the upper digestive tract, encompassing ailments like recurring stomatitis and gastroduodenitis, have long posed challenges, particularly among children. Conventional medical approaches often fall short due to the complex interplay of infection, toxicity, immune imbalances, and autonomic disruptions. However, a groundbreaking solution has emerged – Mycoton, a unique preparation enriched with a chitin, melanin, and glucan complex.
A comprehensive study involving 254 children aged 7 to 15, afflicted with chronic upper digestive tract issues, unveiled Mycoton's transformative potential. These children were grouped based on their underlying microbial causes: zymotic factors, helicobacter, herpesvirus, and candida. Each group underwent distinct treatment regimens, with a subset within each receiving Mycoton alongside their established conventional therapies.
Mycoton's prowess stems from its potent antimicrobial properties, demonstrated in both laboratory and living systems. This preparation harnesses the power of chitin, melanin, and glucan to actively combat microbial infections and absorb a range of toxic agents. In fact, earlier studies highlighted Mycoton's comparable efficacy to traditional antibiotics and antiviral/antifungal drugs.
The results were remarkable. By integrating Mycoton into existing therapeutic strategies, a profound improvement in treating these complex inflammatory conditions was observed. The addition of Mycoton significantly elevated the overall efficacy of the treatment, outperforming conventional therapies alone.
Mycoton offers promise not only in addressing diseases with intricate origins but also those intertwined with infection, toxicity, allergies, and immunodeficiencies. As a beacon of innovation, Mycoton emerges as a prospective game-changer in healing ailments rooted in multifaceted etiologies.
- Addressing Foodborne Intoxication with Selective Enterosorbent Properties
Mycoton, with its remarkable enterosorbent properties, presents a dynamic solution to combat foodborne intoxication. With it’s inherent adsorbent properties, Mycoton possesses the unique ability to selectively target and absorb harmful substances, effectively removing toxins and contaminants from the digestive system. This selective action not only aids in alleviating symptoms of foodborne intoxication but also supports the body's natural detoxification processes. Mycoton stands as an innovative safeguard against the challenges of food-related ailments, offering a path to rapid relief and enhanced well-being.
SAFETY
Extensive research conducted on both laboratory animals as well as humans has demonstrated the remarkable safety profile of Mycoton, even at high doses. Additionally, the impact of Mycoton on patients' internal organs has been thoroughly examined using standard clinical methods. These comprehensive studies have consistently shown no adverse effects on vital organs such as the liver, kidneys, pancreas, peripheral blood, and blood coagulation system. Mycoton is a safe and reliable option for long-term use. In recognition of these findings, the Pharmacological Committee of the Ministry of Public Health Services of Ukraine has granted Mycoton a certification for its non-toxic nature.
RESEARCH
References
1. Белякова Г.А., Дьяков Ю.Т., Тарасов К.Л. Ботаника: в 4 т. - М.: Академия, 2006. - Т. 2. – 320 с.-ISBN 978-5-7695-2750-1.
(Belyakova G.A., Dyakov Yu.T., Tarasov K.L. Botany: in 4 vols. - M.: Academy, 2006. - Vol. 2. - 320 pp. - ISBN 978-5-7695-2750-1.)
2. Гарибова Л.В., Лекомцева С.Н. Основы микологии. - М., 2005. - ISBN 5-87317-265-X.
(Garibova L.V., Lekomtseva S.N. Fundamentals of mycology. - M., 2005. - ISBN 5-87317-265-X.)
3. Бриттон Т. Биохимия природных пигментов / Бриттон Т. – Москва: Мир, 1986. – 422 с.
(Britton T. Biochemistry of natural pigments / Britton T. - Moscow: Mir, 1986. - 422 p.)
4. Chang S.T. Global impact of edible and medicinal mushrooms on human welfare in the 21st century: ngreen revolution // Int. J. Med. Mushrooms. – 1999. – Vol. 1, No 1. – P. 1–7.
5. Бухало А.С. , Соломко Е.Ф., Митропольська Н.Ю. Базидіальні макроміцети з лікарськими властивостями // Український ботанічний журнал. – 1996. – T. 53, № 3. – С.192–200.
(Bukhalo A.S. , Solomko E.F., Mitropolska N.Yu. Basidial macromycetes with medicinal properties // Ukrainian Botanical Journal. – 1996. – T. 53, No. 3. – P.192–200.)
6. Hobbs C. Medicinal mushrooms: An exploration of tradition, healing and culture. 2nd ed. – Santa Cruz Ca: Botanica Press, 1995. – 251 p.
7. Вассер С.П. (ред.) Макромицеты: лекарственные свойства и биологические особенности. – 2012. – Киев, С. 1-285.
(Vasser S.P. (ed.) Macromycetes: medicinal properties and biological features. - 2012. - Kyiv, S. 1-285.)
8. Денисова Н.П. Лечебные свойства грибов. Этномикологический очерк. - СПб.: 1998, С. 1-59.
(Denisova N.P. Medicinal properties of mushrooms. Ethnomycological essay. - St. Petersburg: 1998, S. 1-59.)
9. Филиппова И.А. Популярная фунготерапия: лечение лекарственными грибами. - СПб.: - 2013. – С. 1-128.
(Filippova I.A. Popular fungotherapy: treatment with medicinal mushrooms. - St. Petersburg: - 2013. - S. 1-128.)
10. Dudka I.A. Mushrooms in folk medicine of the eastern slavs // Int. J. Med. Mushr. - 2001. – 3, N 2-3. - P. 135.
11. Л.Ф. Горовой, В.Н. Косяков. Способ получения хитинсодержащего материала. Пат. Российской Федерации № 2073015. МКИ С 37/08 / Приор.11.10.1991.опубл. 10.02.199. – 10 с.
(L.F. Gorovoy, V.N. Kosyakov. Method for obtaining chitin-containing material. Pat. Russian Federation No. 2073015. MKI C 37/08 / Prior.11.10.1991.publ. 02/10/199. – 10 s.)
12. Gorovoj L.F. Mycoton – new chitin materials produced from fungi / Gorovoj L. F., Kosyakov V. N. // Chitin World. Eds. Z. S. Karnicki et al.: proc. 6th International Conference of Chitin and Chitosan. Gdynia, Poland, 16-19 August, 1994. – Wirtschaftsverlag NW, Bremerhaven. – 1995. – P. 632-647.
13. Звіт. Визначення гострої токсичності хітинового перев‘язувального матеріалу мікотон. [наук. кер. д.м.н. Корпачов В. В.] Інститут ендокринології та обміну речовин ім. В. П. Комісаренка АМН України. – 1993.
(Report. Determination of the acute toxicity of the chitin dressing material Mycoton. [science driver Doctor of Medicine VV Korpachev] Institute of Endocrinology and Metabolism named after V. P. Komisarenka of the Academy of Medical Sciences of Ukraine. - 1993.)
14. Muzzarelli R.A. Chitin / Muzzarelli R.A. – Oxford. Pergamon Press, 1977. – 305.p.
15. Pat. DE. 2.923802. Italy, (CL. C 08 B 37/08). Appl. Ital. Chitosan-glucan complex / Muzzarelli, R.A.A. 1978. Publ.1979.
16. Tao Wu. Production and characterization of fungal chitin and chitosan. A thesis presented for the master of science degree. Knoxville: The University of Tennessee, 2004. – 21.p.
17. O’Brien R.W., Ralph B.J. The cell wall composition and taxonomy of some Basidiomycetes and Ascomycetes // Ann. Bot. – 1966. –V. 30, No 120. – P. 831–843.
18. Нудьга Л.А., Ганичева С.И., Петрова В.А. и др. Сорбция ионов Cr (III) хитин-глюкановым комплексов, выделенным из мицелия гриба Aspergillus niger, культивированого в различных условиях // Журн. прикладн. хим. – 1997. – T. 70, № 2. – С. 242–246.
(Nudga L.A., Ganicheva S.I., Petrova V.A. Sorption of Cr (III) ions by chitin-glucan complexes isolated from the mycelium of the fungus Aspergillus niger cultivated under various conditions, Zh. applied chem. - 1997. - T. 70, No. 2. - S. 242-246.)
19. Pumpel T., Schinner F. Native fungal pellits as a biosorbent for heavy metals // FEMS Microbiology reviews. – 1993. – Vol. 11. – P. 159–164.
20. Nishimura S., Ikeuchi Y., Tokura S. // Carbohydrate Res., 1984, N 134, p.305-312.
21. Большаков И.Н., Насибов С.М. Связывание бактериального липополисахарида хитозаном при энтеросорбции в эксперименте /Новые перспективы в исследовании хитина и хитозана. Центр “Биоинженерия” РАН -ВНИРО.Москва-Щелково 25-27 мая 1999 Изд: ВНИРО с.120-122.;
(Bolshakov I.N., Nasibov S.M. Binding of bacterial lipopolysaccharide with chitosan during enterosorption in the experiment / New perspectives in the study of chitin and chitosan. Center "Bioengineering" RAS - VNIRO. Moscow-Shchelkovo May 25-27, 1999 Ed: VNIRO p.120-122.;)
22. Gorovoj L., Burdyukova V., Zemskov V., Prilutsky A. Chitin product Mycoton as an antimicrobic remedy - Polish chitin society - Progress on chemistry and application of chitin and its derivatives /Ed. H.Strusczyk - Vol. 1999 - Lodz, 1999 - P. 7-17.
23. Kobayashi T., Iijima S., Shimada K. A specific absorbent for hepatitis virus. Pat. JP N 105843, 1984.
24. Кочкина З., Чирков С.Н. Влияние хитозана на фаговые инфекции. /Новые перспективы в исследовании хитина и хитозана. Центр “Биоинженерия” РАН-ВНИРО. Москва-Щелково 25-27 мая 1999. Изд: ВНИРО. - с.151-153.
(Kochkina Z., Chirkov S.N. Effect of chitosan on phage infections. / New perspectives in the study of chitin and chitosan. Center "Bioengineering" RAS-VNIRO. Moscow-Schelkovo May 25-27, 1999. Publisher: VNIRO. - pp.151-153.)
25. Azuma I., Iida J., Nishimura K. et al. Stimulation of host mechsnisms with synthetic MDP and chitin derivatives against viral infection in mice. //Prog. Leukocyte Biol. 1987. 6 (Immunopharmacol Infect. Dis.) P. 245-254.
26. Kuprina H., Krasavtsev V. Comparative estimation of bactericidal and sorption properties of chitin and its derivatives being obtained by electrochemical and traditional methods. // In: Advances in chitin science. vol.II. 7th ICCC. FUCHIS’97, Ed. by A.Domard, G.Roberts, K.Varum, Jacques Andre Publisher, 1997, P.914-919;
27. Прилуцкий А., Земсков В., Горовой Л., Бурдюкова Л. /Новые перспективы в исследовании хитина и хитозана. Центр “Биоинженерия” РАН -ВНИРО. Москва-Щелково 25-27 мая 1999. - Изд: ВНИРО. - с.181-186.
(Prilutsky A., Zemskov V., Gorovoy L., Burdyukova L. / New perspectives in the study of chitin and chitosan. Center "Bioengineering" RAS - VNIRO. Moscow-Schelkovo May 25-27, 1999. - Ed: VNIRO. - pp.181-186.)
28. Kogan G., Machova E., Chorvatovicova D., Slovacova L. et al. Chitin-glucan complex of Aspergillus niger and its derivatives: antimutagenic, antiinfective and antiviral activity //In: Advances in chitin science. vol.II. 7th ICCC. FUCHIS’97, Ed. by A.Domard, G.Roberts, K.Varum, Jacques Andre Publisher. - 1997. - p.640-647;
29. Nishimura K., Ichiro A. Immunomodulating activities of chitin derivaties. In: Chitin derivatives in life science. Ed by Tocura S., Azuma I., Org. Committee of international Symposium on Chitin Derivatives in Life Sciences and Japanese Society for Chitin/Chitosan: 1992, p.7-11;
30. Nishimura K., Nishimura S., Saiki I. et al. Immunological activity of chitin and its derivatives. //Vaccine. 1984, v.2., N1. p.93-99;
31. Nishimura K., Nishimura S., Seo H. et al. Effect of multiporous micrpospheres derived from chitin and partially deacetylated chitin on the activation of mouse peritoneal macrophages //Vaccine. 1987. v.5. N2. p. 136-140;
32. Nishimura S., Nishi N.. Tokura S. et al. Bioactive chitin derivatives. Activation of mouse-peritoneal macrophages by O-(carboxymethyl) chitins. // Carbohydr. Res. - 1986. -V.146, N 2. - p. 251-258;
33. Nishimura K., Ichiro A. Immunomodulating activities of chitin derivaties. In: Chitin derivatives in life science. Ed by Tocura S., Azuma I., Org. Committee of international Symposium on Chitin Derivatives in Life Sciences and Japanese Society for Chitin/Chitosan: 1992. - p.7-11;
34. Сенюк О.Ф., Горовой Л.Ф., Трутнева И.А. Использование хитинового препарата микотон в качестве радиопротектора / Новые перспективы в исследовании хитина и хитозана. Центр “Биоинженерия” РАН -ВНИРО.Москва-Щелково 25-27 мая 1999. - : ВНИРО. - с.193 -197;
(Senyuk O.F., Gorovoy L.F., Trutneva I.A. The use of the chitin preparation Mycoton as a radioprotector / New perspectives in the study of chitin and chitosan. Center "Bioengineering" RAS - VNIRO. Moscow-Schelkovo May 25-27, 1999. -: VNIRO. - p.193 -197;)
35. Gonzalez-Davila M., Santana-Casiano J. M., Millero F. J. The adsorption of cadmium and lead (II) to chitin in seawater // J. Colloid. Interface Sci. – 1990. – Vol. 137, No 1. – P. 102–110.
36. Ashworth I.J., Amin J.V., Ashworth I.J.A mechanism for mercury tolerance in fungi // Phytopathology. – 1964. –Vol. 54. – P. 1459–1463.
37. G. Venkateswerlu, Sivarama Sastry K. The mechanism of uptake of cobalt ions by Neurospora crassa // Biochem. J. - 1970. – Vol. 118. – P. 497–503.
38. Duddridge J.I., M. Wainwright Heavy metal accumulation by aquatic fungi and reduction in viability of Gammarus Pulex fed Cd2+ contaminated mycelium // Woter Research. –1980. – Vol. 14. – P. 1605–161.
39. Muzzarelli R.A.A., R. Roccetti, G. Marangio. Separation of zirconium, niobium, ceriumand ruthenium for the determination of cesium in nuclear fuel solution //Radional. Chem. – 1972. – Vol. 10. – P. 17–26.
40. Muzzarelli R.A.A., Tanfani F., Scarpini G. Chelating, film-forming, and coagulating ability of the chitosan-glucan complex from Aspergillus niger industrial wastes //Biotechnology and Bioengineering. – 1980. – Vol.23. – P. 885–896.
41. Горовой Л.Ф., Косяков В.Н. Клеточная стенка грибов – оптимальная структура для биосорбции // Биополимеры и клетка. – 1996. –Т. 12., № 4. – С.49–60.
(Gorovoy L.F., Kosyakov V.N. The cell wall of fungi is the optimal structure for biosorption // Biopolymers and cells. - 1996. -T. 12., No. 4. - P. 49–60.)
42. Gorovoj L., Kosyakov V. Chitin and chitosan biosorbents for radionuclides and heavy metals. In: Advances in chitin scince. V.2. Proc. 7th ICC.A. Lion. France. Ed. By Domard et al. – Lion: Jacques Andre,1997. – P. 858–863.
43. Косяков В.Н., Яковлев Н.Г., Велешко И.Е. и др. Сорбция актиноидов на хитиновых сорбентах волокнистой структуры //Радиохимия. – 1997. – Т. 39, № 6. – С.540–543
(Kosyakov V.N., Yakovlev N.G., Veleshko I.E. and others Sorption of actinoids on chitinous sorbents with a fibrous structure //Radiochemistry. - 1997. - T. 39, No. 6. – P.540–543)
44. Горовой Л.Ф., Косяков В.Н. Сорбционные свойства хитина и его производных. Хитин и хитозан. Получение, свойства и применение. Ред. Скрябин К.Г., Вихорева Г.А., Варламов В.П. Москва, Наука, 2002. С. 217-246.
(Gorovoy L.F., Kosyakov V.N. Sorption properties of chitin and its derivatives. Chitin and chitosan. Obtaining, properties and application. Ed. Skryabin K.G., Vikhoreva G.A., Varlamov V.P. Moscow, Nauka, 2002, pp. 217-246.)
45. Прилуцький О.І., Горовий Л.Ф., Земсков С.В. Застосування хітинового сорбенту Мікотон для активної детоксикації організму в хірургічному лікуванні хворих з механічною жовтяницею непухлинного генезу //Укр. наук.-мед. молодіж. журн. – 1997. – № 4. – С. 26–29.
(Prylutsky O.I., Horovy L.F., Zemskov S.V. The use of chitin sorbent Mycoton for active detoxification of the body in the surgical treatment of patients with mechanical jaundice of non-neoplastic origin //Ukr. science and medicine youth journal – 1997. – No. 4. – P. 26–29.)
46. Сенюк О.Ф., Сенюк Х.В., Горовой Л.Ф. Перспективы использования препарата микотон для лечения почечной недостаточности //Успехи медицинской микологии. Том 1. Материалы первого всероссийского конгресса по медицинской микологии. – Москва: Национальная академия микологии, 2003. – С. 296–297.
(Senyuk O.F., Senyuk H.V., Gorovoy L.F. Prospects for the use of the drug Mycoton for the treatment of renal failure //Advances in Medical Mycology. Volume 1. Materials of the first All-Russian Congress on Medical Mycology. - Moscow: National Academy of Mycology, 2003. - S. 296–297.)
47. Сенюк Х.В. Ефективність хітин-вмісного препарата мікотон в комплексному лікуванні хворих хронічним гломерулонефритом: магістерська робота …просвітницько-кваліфікаційний рівень «магістр медицини»: 14.01.02. – Київ, 2002. – 39 с.
(Senyuk H.V. The effectiveness of the chitin-containing drug Mycoton in the complex treatment of patients with chronic glomerulonephritis: master's thesis ... educational and qualification level "master of medicine": 14.01.02. - Kyiv, 2002. - 39 p.)
48. Ruiz-Herrera J. Biosynthesis of -glucans in fungi //Antonie van Leeuwenhoek. – 1991. – Vol. 60, No 2. – P. 73–81.
49. Mizuno T. The extraction and development of antitumoractive polysaccharides from medicinal muschrooms in Japan (Review) //Int. J.Medicinal muschrooms.- 1999.- V.1, N1.- P. 9-29.
50. Феофилова Е.П. Клеточная стенка гpибов / – Москва: Наука,1983. –248 с.
(Feofilova E.P. The cell wall of fungi / - Moscow: Nauka, 1983. –248 s.)
51. Бадалян С.М. Основные группы и терапевтическая значимость биоактивных метаболитов, образуемых макромицетами //Проблемы медицинской микологии. –2000. – T. 3, № 1. – С. 16–23.
(Badalyan S.M. The main groups and therapeutic significance of bioactive metabolites formed by macromycetes // Problems of medical mycology. –2000. - T. 3, No. 1. - P. 16–23.)
52. Lindequist U., Niedermeyer T.H.J., Julich W.-D. The pharmacological potential of mushrooms // CAM. – 2005. – Vol. 2, No 3. – P.285–299.
53. Brandt C.R., Pirano F. Mushroom antiviral //Recent Res. Dev. Antimicrobial Agents Chemotherapy. – 2000. – No 4. – P. 11–26.
54. Seniuk O.F., Gorovoj L.F., Beketova G.V. et al. Anti-Infective Properties of Melanin-Glucan Complex Obtained from Medicinal Tinder Bracket Mushroom, Fomes fomentarius L.: Fr.) F. Aphyllophormyetideae //Int. J. Med.l Mushrooms. – 2011. – Vol. 1. – P. 7–18.
55. Kaning Wong C., Connie K.M.L., Peter C.K. Molecular mechanisms of immunomodulation of immune cells by mushroom β-glucans /Proceedings of the 5th International Medicinal Mushroom Conference. 5th-8th September 2009. – Nantong, China, 2009. – P. 63–71.
56. Rowan N.J., John E. Smith, Richard Sullivan Immunomodulatory activities of mushroom glucans and polysaccharideprotein complexes in animals and humans (a review) // Int. J. Med. Mushrooms. – 2003. – Vol. 5. – P. 95–110.
57. Smith J.E., Richard S., Neil R. The role of polysaccharides derived from medicinal mushrooms in cancer treatment programs: current perspectives (review) //Int. J. Med. Mushrooms. – 2003. – Vol.5, No3. – P. 217–234.
58. Seniuk O.F. Leontiy F. Gorovoj L.F., Kovalev V.O. et al. Anticarcinogenic properties of melanin-glucan complex from Higher fungi /Proceedings of the 5th International Medicinal Mushroom Conference. 5th-8th September 2009. – Nantong, China, 2009. – Р. 142–149.
59. Soltys J., Benkova M., Boroskova Z. Influence of glucan on the complement level in experimental ascariosis //Helminthologia. – 1992. – Vol. 29, No 2. – P. 93–96.
60. Chmielnicka J., Mlodecki H., Trocha M. Enzymy chitynolityczne (chitynaza i chitobiaza) w grzybach // Acta mycol. – 1970. – Vol. 6, No 2. – P. 315–323.
(Chmielnicka J., Mlodecki H., Trocha M. Chitinolytic enzymes (chitinase and chitobiase) in mushrooms // Acta mycol. – 1970. – Vol. 6, No. 2. – P. 315–323.)
61. Даниляк М.І., Решетніков С.В. Лікарські гриби. Медичне застосування та проблеми біотехнології / Київ: Інститут ботаніки НАН України, 1996. – (Препр. / НАН України, Інститут ботаніки, 1996 – 63 с).
(Danylyak M.I., Reshetnikov S.V. Medicinal mushrooms. Medical application and problems of biotechnology / Kyiv: Institute of Botany of the National Academy of Sciences of Ukraine, 1996. - (Prepr. / National Academy of Sciences of Ukraine, Institute of Botany, 1996 - 63 p.).)
62. Жданова Н.Н. Василевская А.И. Меланинсодержащие грибы в экстремальных условиях – Київ: Наук. думка, 1988. – 196 с.
(Zhdanova N.N. Vasilevskaya A.I. Melanin-containing fungi in extreme conditions - Kiev: Nauk. Dumka, 1988. - 196 p.)
63. Yang Q.Y., Jong S.C. Medicinal mushrooms in China // Mushroom Science XII (Part 1). – 1989. – P. 631–643.
64. Swan G.A. Chemical structure of melanins //Ann. N.Y. Academy Sciences. – 1963. – Vol. 100. – P. 1005–1019.
65. Urabe K., Aroca P., Hearing V.J. From gene to protein: determination of melanin synthesis //Pigment Cell Res. – 1993. – Vol. 6, No 4. – P. 186–192.
66. Almendros G., Martin F., Gonzalez-Vila F.J. et al. Melanins and lipids in Lycoperdon perlatum fruit bodies //Trans. Brit. Mycol. Soc. – 1987. – Vol. 89, No 4. – P. 533–537.
67. Bloomfield B.J., Alexander M. Melanins and resistance of fungi to lysis //J. Bacteriol. – 1967. – Vol. 93, No 4. – P. 1276–1280.
68. Kuo M.J. Alexander M. Inhibition of lysis of fungi by melanins // J. Bacteriol. – 1967. – Vol. 94. – P. 624–629.
69. Nicolaus G.L. Melanins – Paris: Herman, 1968. – 127 p.
70. Hegnauer H., Nyhlen L.E., Rast D.M. Ultrastructure synthetic mentation of melanogenesis in fungi //Exp. Mycol. – 1985. Vol. 9, No 3. – P. 221–229.
71. Pierce J.A., Rast D.M. Permanganate degradation of a fungal melanin and characterization of the decomposition products by infrared spectrometry / Pierce J.A., //Physiol. Chem. Phys. Med. NMR. – 1991. – Vol. 23, No 3. –P. 161–166.
72. Щерба В.В., Бабицкая В.Г., Курченко В.П. и др. Антиоксидантные свойства меланиновых пигментов грибного происхождения //Прикладная биохимия и микробиология. – 2000. – Т. 36, № 3. – С. 569–574.
(Shcherba V.V., Babitskaya V.G., Kurchenko V.P. and other Antioxidant properties of melanin pigments of fungal origin //Applied Biochemistry and Microbiology. - 2000. - T. 36, No. 3. - S. 569-574.)
73. Жданова Н.Н., Борщевская М.И. Биомелан – лечебный препарат грибного происхождения //Современная микология в России: мат. 1-го съезда микологов России : тезисы докл. – Москва, 2002. – С. 259.
(Zhdanova N.N., Borshchevskaya M.I. Biomelan - a medicinal preparation of fungal origin // Modern mycology in Russia: mat. 1st Congress of Mycologists of Russia: abstracts of reports. - Moscow, 2002. - S. 259.)
74. Riley P.A. Melanin. //Int J Biochem Cell Biol. –1997. – Nov;29(11). – P.:1235-1239.
75. Лях С.П. Микробный меланиногенез и его функции – Москва: Наукa,1981. – 274 с.
(Lyakh S.P. Microbial melaninogenesis and its functions - Moscow: Nauka, 1981. – 274 p.)
76. Бабицкая В.Г., Щерба В.В. Природа меланиновых пигментов некоторых микро- и макромицетов // Прикладная биохимия и микробиология. – 2002. – T. 38, № 3. – С. 286–291.
(Babitskaya V.G., Shcherba V.V. The nature of melanin pigments in some micro- and macromycetes // Applied Biochemistry and Microbiology. - 2002. - T. 38, No. 3. - S. 286-291.)
77.Жеребин Ю.Л. Сава В.М., Богатский А.В. Антиокислительная активность эномеланинов //Журн. общ. химии. – 1981. – Т. 51, № 12. – С. 2767–2773.
(Zherebin Yu.L. Sava V.M., Bogatsky A.V. Antioxidant activity of enomelanins // Zhurn. total chemistry. - 1981. - T. 51, No. 12. - S. 2767-2773.)
78. Коржова Л.П., Фролова Е.В., Ромаков Ю.А. и др. Фотодеструкция синтетического ДОФА-меланина // Биохимия. – 1989. – Т. 54, № 6. – С. 992–998.
(Korzhova L.P., Frolova E.V., Romakov Yu.A. et al. Photodestruction of synthetic DOPA-melanin // Biochemistry. - 1989. - T. 54, No. 6. - S. 992-998)
79. Моссе И.Б. Радиация и наследственность: Генетические аспекты противо-радиационной защиты. – Минск: Университетское, 1990. – 208 с.
(Mosse I.B. Radiation and heredity: Genetic aspects of anti-radiation protection. - Minsk: University, 1990. - 208 p.)
80. Моссе И.Б., Кострова Л.Н., Дубовик Б.В. и др. Влияние меланина на мутагенное действие хронического облучения и адаптивный ответ у мышей // Радиац. биол. Радиоэкол. – 1999. – Т. 39, №2–3. – С.329–333.
(Mosse I.B., Kostrova L.N., Dubovik B.V. Influence of melanin on the mutagenic effect of chronic irradiation and adaptive response in mice // Radiats. biol. Radioecol. - 1999. - V. 39, No. 2–3. – P.329–333.)
81. Изместьева О.С., Дубовик Б.Г., Жаворонков Л.П. и др. Экспериментальная оценка радио-защитного действия меланина на соматическое развитие при облучении в антенатальном периоде онтогенеза //Радиац. биол. Радиоэкол. – 2007. – Т. 46. № 6. – С.684–689.
(Izmest'eva O.S., Dubovik B.G., Zhavoronkov L.P. and others. Experimental evaluation of the radio-protective effect of melanin on somatic development during irradiation in the antenatal period of ontogenesis // Radiats. biol. Radioecol. - 2007. - T. 46. No. 6. - P. 684-689.)
82. Сидорик Є.П., Дружина М.О., Бурлака А.П. и др. Регуляторний вплив меланіну грибного походження на швидкість генерування і вміст радикальних форм кисню у мікросомах печінки тварин, які знаходились тривалий час у зоні аварії на Чорнобильській АЕС // Доклады Академии наук Украины. 1994. № 9. С. 174-176.
(Sydorik E.P., Wife M.O., Burlaka A.P. et al. Regulatory influence of melanin of fungal origin on the rate of generation and content of oxygen radical forms in liver microsomes of animals that were in the Chernobyl NPP accident zone for a long time // Doklady Akademii Nauk Ukrainy. 1994. No. 9. P. 174-176.)
83. Rashydov N. Seniuk O., Gorovyy L., et al. /Nuclear Power Plants; ed. by S. H. Chang. – Сroatia : INTECH, 2012. – P. 231–278.
84. Сенюк О.Ф., Горовий Л.Ф., Паламар Л.А. Радіопротекторний вплив на ДНК мишей комплексів біополімерів з трутовика Fomes fomentarius за дії іонізуючих випромінювань у малих дозах //Ядерна фізика та енергетика. – 2014. – Т. 15, №1. – С.73–81.
(Senyuk O.F., Horovy L.F., Palamar L.A. Radioprotective effect on mice DNA of biopolymer complexes from Fomes fomentarius under the action of ionizing radiation in small doses // Nuclear physics and energy. – 2014. – Vol. 15, No. 1. – P.73–81.)
85. Сенюк О.Ф., Ковальов О.В., Паламар Л.А. Радіозахисні ефекти меланін- глюкан-хітиновового комплексу з трутовика Fomes fomentarius і індраліну при опроміненні мишей BALB/c дозою 5,95 Гр/8,5 хв //Ядерна фізика та енергетика. – 2014. – Т. 15, № 2. – С. 178–188.
(Senyuk O.F., Kovalev O.V., Palamar L.A. Radioprotective effects of the melanin-glucan-chitin complex from the tinder plant Fomes fomentarius and indralin upon irradiation of BALB/c mice with a dose of 5.95 Gy/8.5 min //Nuclear Physics and Energy. – 2014. – Vol. 15, No. 2. – P. 178–188.)
86. Cенюк О.Ф., Горовой Л.Ф., Курченко В.П. Защита генома человека от низкоинтенсивного радиационного и химического факторов // Экологическая антропология. Ежегодник: сб. научн. трудов основ. 1996 г. / ред. Е.Ф. Конопля. – Минск: Белорусский комитет «Дзеці Чарнобыля». – 2005. – С.205–209.
(Senyuk O.F., Gorovoy L.F., Kurchenko V.P. Protection of the human genome from low-intensity radiation and chemical factors // Ecological Anthropology. Yearbook: Sat. scientific works of the basics. 1996 / ed. E.F. Hemp. - Minsk: Belarusian Committee "Children of Chernobyl". - 2005. - S.205-209.)
87. Сенюк О.Ф., Ковалев В.А., Круль Н.И. и др. Дистанционная передача сигналов лучевого поражения в межклеточном пространстве в организме у мышей с различным уровнем генетически детерминированной радиочувствительности //Радиац. биол. Радиоэкол. – 2013. – Т.53, №1. – С. 33–46.
(Senyuk O.F., Kovalev V.A., Krul N.I. Remote transmission of radiation damage signals in the intercellular space in mice with different levels of genetically determined radiosensitivity // Radiats. biol. Radioecol. - 2013. - T.53, No. 1. – P. 33–46.)
88. Сенюк О.Ф., Горовой Л.Ф., Ковалев В.А. и др. Особенности и возможность химической модификации поведенческих реакций в приподнятом крестообразном лабиринте хронически облученных мышей с различной генетически детерминированной радиочувствительностью // Радиац. биол. Радиоэкол. – 2013. –Т. 53, № 2. – С. 170–182.
(Senyuk O.F., Gorovoy L.F., Kovalev V.A. Peculiarities and possibility of chemical modification of behavioral responses in the elevated plus maze of chronically irradiated mice with different genetically determined radiosensitivity // Radiats. biol. Radioecol. - 2013. -T. 53, No. 2. - P. 170-182.)
89. Сенюк О.Ф, Горовой Л.Ф., Данилов В.М. Радиопротекторы нового поколения для радиационной защиты персонала в условиях современного объекта «Укрытие» //Проблеми Чорнобиля. –2001. – Вип. 7. – С. 219–229.
(Senyuk O.F., Gorovoy L.F., Danilov V.M. Radioprotectors of a new generation for radiation protection of personnel in the conditions of a modern Shelter object // Problems of Chernobyl. -2001. - Vip. 7. - S. 219-229.)
90. Сенюк О.Ф., Л.Ф. Горовой, В.П. Варламов и др. Влияние хитозана и меланин-глюканового комплекса на последствия острого гамма-облучения в малых дозах и стрессовой реакции //Проблеми Чорнобиля. – 2004. – Вип. 15. – С. 121–126.
(Senyuk O.F., L.F. Gorovoy, V.P. Varlamov et al. Influence of chitosan and melanin-glucan complex on the consequences of acute gamma irradiation in small doses and stress response // Problems of Chernobyl. - 2004. - VIP. 15. - S. 121-126.)
91. Горовой Л.Ф., Косяков В.Н., Велешко И.Е. и др. Радиосорбционные свойства хитин-меланиновых комплексов и перспективы их использования в радиационной защите //Проблеми безпеки атомних електростанцій і Чорнобиля. – 2005. – Вип. 3, Ч. 1. – С. 140–150.
(Gorovoy L.F., Kosyakov V.N., Veleshko I.E. Radiosorption properties of chitin-melanin complexes and prospects for their use in radiation protection // Problems of safety of nuclear power plants in Chernobyl. - 2005. - VIP. 3, Part 1. - P. 140–150.)
92. Senyuk O.F., Gorovoj L.F., Zhidkov A.V. et al. Genome protection properties of the chitin-containing preparation Mycoton /Advances in Chitin Science. Proc. of 6rd Intern. Conf. European Chitin Society, Poznan, Poland Aug. 31–Sept. 3, 2004. еd. by H. Struszczyk, M. G. Peter, A Domard. – Poznan: ESUS, 2005. – Vol. VIII. – P. 430–439.
93. Курченко В.П., Сенюк О.Ф., Горовой Л.Ф. и др. Антиоксидантные и генопротекторные свойства природных меланиновых пигментов: материалы Междунар. научн. конф. «Биохимия биологически активных соединений», 16–18 нояб. 2005 г., Минск // Биохимия. Минск: РИВШ, 2005. – С. 86–93.
(Kurchenko V.P., Senyuk O.F., Gorovoy L.F. and others. Antioxidant and gene-protective properties of natural melanin pigments: materials of the Intern. scientific conf. "Biochemistry of biologically active compounds", November 16–18. 2005, Minsk // Biochemistry. Minsk: RIVSh, 2005. - S. 86–93.)
94. Сенюк О.Ф., Горовой Л.Ф., Варламов В.П. Хитозан и меланин-глюкановый комплекс в защите от последствий гамма-облучения: Материалы Восьмой Международной конференции «Современные перспективы в исследовании хитина и хитозана», 12–17 июн. 2006 г., Казань. – Москва : ВНИРО, 2006. – С.243–248.
(Senyuk O.F., Gorovoy L.F., Varlamov V.P. Chitosan and the melanin-glucan complex in protection against the effects of gamma irradiation: Proceedings of the Eighth International Conference "Modern Perspectives in the Study of Chitin and Chitosan", June 12–17. 2006, Kazan. - Moscow: VNIRO, 2006. - P. 243–248.)
95. Дружина М.О., Пухова Г.Г., Бурлака А.П. та ін. Процеси перекисного окислення в системі крові та їх корекція меланіном у тварин в зоні впливу аварії на Чорнобильській АЕС //Український радіологічний журнал. – 1994. – Т.ІІ, Вип. 4. – С. 274–273.
(Druzhyna M.O., Pukhova H.G., Burlaka A.P. etc. Processes of peroxidation in the blood system and their correction by melanin in animals in the zone affected by the accident at the Chernobyl NPP //Ukrainian Radiological Journal. – 1994. – Volume II, Issue 4. – pp. 274–273.)
96. Борщевская М.И., Васильева С.М. Развитие представлений о биохимии и фармакологии меланиновых пигментов //Вопросы медицинской химии. – 1999. – № 1. – С. 13–23.
(Borshchevskaya M.I., Vasilyeva S.M. Development of ideas about the biochemistry and pharmacology of melanin pigments // Issues of medical chemistry. - 1999. - No. 1. - P. 13–23.)
97. Fogarty R.V., Tobi J.M. Fungal melanins and their interactions with metals //Enzyme Microb. Technol. – 1996. – Vol. 19. – P. 311–317.
98. Запрометова Л.М., Мирчинко Т.Г Микробные метаболиты. Пигменты темноокрашенных грибов и их экологическая роль – Москва: МГУ, 1979. – С. 193–209.
(Zaprometova L.M., Mirchinko T.G. Microbial metabolites. Pigments of dark-colored fungi and their ecological role - Moscow: Moscow State University, 1979. - P. 193–209.)
99. Велешко А.Н. Розанов К.В., Велешко И.Е. и др. Комплексообразование урана (vi) препаратами из природных биополимеров / Мат. XVIII-го Менделеевского съезда по общей и прикладной химии: Тез. докл. – Москва, 2007. – С. 211.
(Veleshko A.N. Rozanov K.V., Veleshko I.E. Uranium (vi) complexation with preparations from natural biopolymers / Mat. XVIII-th Mendeleev Congress on General and Applied Chemistry: Proceedings. report - Moscow, 2007. - S. 211.)
99. Veleshko A., K. Rosanov et al. Application of bioadsorption process for radioactive and toxic liquid wastes treatment / Berlin-Brandenburgischen Forschun-symposium. "Biomassebasierte Wertschoepfungsketten und Weisse Biotechnologie–Quellen fuer die Entwicklung innovativer Produkte und Technologien". Abstracts. – Biotechnologiepark Luckenwalde, 2007. – P. 25–26.
(Berlin-Brandenburg Research Symposium. "Biomass-based value chains and white biotechnology - sources for the development of innovative products and technologies". abstracts. - Luckenwalde Biotechnology Park, 2007. - P. 25-26.)
100. Сенюк О.Ф., Горовой Л.Ф., Паламар Л.А. и др. Влияние меланин-глюканового комплекса, выделенного из грибов трутовика, на продолжительность самок мышей линии ICR //Проблемы старения и долголетия. – 2014. – Т. 23, № 1. – С. 11–27.
(Senyuk O.F., Gorovoy L.F., Palamar L.A. Influence of the melanin-glucan complex isolated from tinder fungus on the duration of ICR female mice //Problems of aging and longevity. - 2014. - V. 23, No. 1. - S. 11–27.)
101. O.F. Seniuk, L.F. Gorovoj, G.V. Beketova et al. AntiInfective Properties of Melanin-Glucan Complex Obtained from Medicinal Tinder Bracket Mushroom, Fomes fomentsrius (L.: Fr.) Fr. (Aphyllophoromycetideae). / Int. J. Medicinal Mushrooms. – 2011. – Vol. 13, # 1. – P. 7–18.
102. П.Г. Рытик, И.И. Кучеров, Л.Ф. Горовой, О.Ф. Сенюк, Л.О. Мистрюкова, И.А. Подольская Природные ингибиторы репликативной активности ВИЧ //Материалы XI съезда гигиенистов и эпидемиологов РБ. – Минск, 16 ноября 2007 г. – Минск. – 2007. – С. 281-286.
(P.G. Rytik, I.I. Kucherov, L.F. Gorovoy, O.F. Senyuk, L.O. Mistryukova, I.A. Podolskaya Natural inhibitors of HIV replication activity // Materials of the XI Congress of hygienists and epidemiologists of the Republic of Belarus. - Minsk, November 16, 2007 - Minsk. - 2007. - S. 281-286.)
103. O’Brien P. Multiple mechanisms of metabolic activation of arilamone cancerogens // Free Radicals in Biology. Ed. W. Pryor. – 1984. –Vol. 6. – 314 p
104. O. Senyuk, L. Gorovoj, V. Kurchenko et al. Genome protection properties of the melanin-containing complexes from the Hygher Basidiomycetes. Current problems of radiation research /Proc. of 35th Annual Meeting of the European Radiation Research Society [Current problems of radiation research], Kiev, Ukraine, Aug 23–25, 2006 : ed. by D. Grodzinsky, A. Dmitriev. – Kiev, 2007. – P. 224–239.
105. Wasser S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl.Microbiol.Biotechnol. 2002, 60, P.258-274.
106. Zjawiony J.K. Biologically active compounds from Aphyllophorales (Polypore) fungi. J. Nat. Prod. 2004, 67, P. 300-310.
107. Elmendorff-Dreikorn K, Chauvin C., Slor H., et al. Assessment of DNA damage and repair in human peripheral blood mononuclear cells using a novel DNA unwinding technique // Сellular and Molecular Biology. 1999.45(2), 211-218;
108. Seniuk O.F., Gorovoj L.F., Kovalev V.A. et al. Anticarcinogenic Properties of Melanin-Glucan complex from Higher Fungi /Proc. of the 5th International Medicinal Mushroom Conference, 5th–8th Sept. 2009, China, Nantong.: ed. by Wasser S. P. – Nantong, 2009. – P.142–149.
109. Nagasawa M, Little J.B. Induction of sister chromatid exchange by extremely low doses of alfa-particles // Cancer Res. -1992, 52.H.6394-6396.
110. Marnett L.J. Oxyradicals and DNA damage //Carcinogenesis: Oxford, 2000. V. XXI. N3. P.361-370.
111. Ames B.N. Endogenous DNA damage as related to cancer and aging //Mutat.Res. 1989. V. 214. -P. 41.
112. О.Ф. Сенюк, В.А. Ковалев, А.В. Жидков и др. «Эффект свидетеля» при остром внешнем облучении Balb/c мышей. /Материалы IV международной научно-практической конференции. 11-12 апреля 2007 г. Северск-Томск, 2007, с. 150-151.
(O.F. Senyuk, V.A. Kovalev, A.V. Zhidkov et al. “Witness effect” in acute external irradiation of Balb/c mice. /Materials of the IV international scientific-practical conference. April 11-12, 2007 Seversk-Tomsk, 2007, p. 150-151.)
113. Krawitt E.L. Autoimmune Hepatitis //The New England Journal of Medicine. – 1996. – 334, №14. – P. 897–903.
114. Aprosina Z.G. Autoimmune Hepatitis //Russian Gastroenterology, Hepatology and Coloproctology Journal. – 1998. – №5. – P. 47–56.
115. Buskila D., Sikuler E., Shoenfeld Y. Hepatitis C Virus and Elselvier Autoimmunity //The decade of autoimmunity. – 1999. P. 355-363.
116. Manns M, Gerken G, Kyriatsoulis A, Staritz M, Meyer zum Büschenfelde K-H. Characterisation of a new subgroup of autoimmune chronic active hepatitis by autoantibodies against a soluble liver antigen // Lancet. – 1987. – Р. 292 – 294.
117. E. Ballot, J. C. Homberg, C. Johanet. Antibodies to soluble liver antigen: an additional marker in type 1 autoimmune hepatitis //J. Hepatology, – 2000. – 33, – P. 208 – 215.
118. Manns M.P. Autoantibodies in chronic hepatitis: diagnostic reagents and cell biology //Progress in Liver Diseases. – 1995. – P. 137–156.
119. Treichel U., Schreiter T, Zumbuschenfelde K.H.M., et al. Purification and Characterization of Human Asialoglycoprotein Receptor // Protein Expression and Purification –1995. – 6, № 3. – Р. 251–255.
120. Uibo R.M., Helin H.J., Krohn K.J. Immunological reactions with membrane liver-specific lipoprotein (LSP) in experimental autoimmune damage of liver in rabbits // Clin. Exp. Immunol. –1982. – 48, № 2. – P. 505 –512.
121. Garcia-Buey., Garcia-Monzon C., Rodriguez S., et al. Latent autoimmune hepatitis triggered during interferon therapy in patients with chronic hepatitis C //Gastroenterology –1995. – №.108. –Р. 1770-1777.
122. Chung Y.H., Shong Y.K. Development of thyroid autoimmunity after administration of recombinant human interferon-alpha 2b for chronic viral hepatitis //Am. J. Gastroenterol. –1993. –№88. – Р. 244 –247.
123. Wesierska-Gadek J., Grimm R., Hitchman E., Penner E. Members of the glutathione S-transferase gene family are antigens in autoimmune hepatitis //Gastroenterology. – 1998. – 114, №2. – P. 329-335.
124. Ковалев В.А., Сенюк О.Ф. Состояние толерантности в условиях воздействия ионизирующих излучений «чернобыльского спектра» //Экологический вестник. – 2008. – Минск. – Вып. 5, № 2. – С 36–43.
(Kovalev V.A., Senyuk O.F. The state of tolerance under the influence of ionizing radiation of the "Chernobyl spectrum" // Ecological Bulletin. - 2008. - Minsk. - Issue. 5, No. 2. - From 36–43.)
125. Сенюк О.Ф., Курочко Н.Ф., Горовой Л.Ф.. Использование грибного препарата Микотон в лечении пациентов старческого возраста //Иммунопатология, аллергология, инфектология. - № 1 – 2010 – С. 266
(Senyuk O.F., Kurochko N.F., Gorovoy L.F. The use of the mushroom preparation Mycoton in the treatment of senile patients // Immunopathology, allergology, infectology. - No. 1 - 2010 - P. 266)
126. Вовк А.Д., Соляник И.В., Сенюк О.Ф. и др. Клиническая эффективность микотона при лечении хронических гепатитов С /Успехи современной микологии. Под ред. Ю.В. Сергеева Материалы четвертого всероссийского конгресса по медицинской микологии Т.VII, Изд. Москва, Национальная Академия Микологии, 2006, с.183-185.
(Vovk A.D., Solyanik I.V., Senyuk O.F. et al. Clinical efficacy of Mycoton in the treatment of chronic hepatitis C /Advances in modern mycology. Ed. Yu.V. Sergeeva Proceedings of the Fourth All-Russian Congress on Medical Mycology T.VII, Izd. Moscow, National Academy of Mycology, 2006, pp. 183-185.)
127. Наконечная А.А., Дранник Г.Н., Горовой Л.Ф. и др. Клинико-иммунологическая эффективность хитинового препарата Микотон в комплексном лечении хронического гепатита. Центр “Биоинженерия” РАН-ВНИРО. Москва-Щелково 25-27 мая 1999. - Изд: ВНИРО. - с.172-175.
(Nakonechnaya A.A., Drannik G.N., Gorovoy L.F. Clinical and immunological efficacy of the chitinous drug Mycoton in the complex treatment of chronic hepatitis. Center "Bioengineering" RAS-VNIRO. Moscow-Schelkovo May 25-27, 1999. - Ed: VNIRO. - pp.172-175.)
128. Сенюк О.Ф., Сенюк Х.В., Горовой Л.Ф. Перспективы использования препарата микотон для лечения почечной недостаточности. Успехи медицинской микологии. Том 1. Материалы первого всероссийского конгресса по медицинской микологии. Москва: Национальная академия микологии. 2003, с. 296-297.
(Senyuk O.F., Senyuk H.V., Gorovoy L.F. Prospects for the use of the drug Mycoton for the treatment of renal failure. Advances in medical mycology. Volume 1. Materials of the first All-Russian Congress on Medical Mycology. Moscow: National Academy of Mycology. 2003, p. 296-297.)
129. Бекетова Г.В., Савичук Н.О., Сенюк О.Ф. и др. Клиническая эффективность микотона в лечении хронических поражений верхних отделов пищеварительного тракта. Успехи медицинской микологии. Том 1. Материалы первого всероссийского конгресса по медицинской микологии. Москва: Национальная академия микологии. 2003, с. 229-230.
(Beketova G.V., Savichuk N.O., Senyuk O.F. Clinical efficacy of Mycoton in the treatment of chronic lesions of the upper digestive tract. Advances in medical mycology. Volume 1. Materials of the first All-Russian Congress on Medical Mycology. Moscow: National Academy of Mycology. 2003, p. 229-230.)
https://www.dl.begellhouse.com/journals/708ae68d64b17c52,2e5fc0e3182d70db,7db4481501fea282.html
130. L.Gorovoj, O. Senyuk, G. Beketova et al. Chitosan per os: from dietary supplement to drug carrier. Ed. by R.A.A. Muzzarelli. – Ancona: Atec, 2000. – P.201–221.
131. Seniuk O.F., Gorovoy L.F., Kurochko N.F. Use of Mushroom Preparation Mycoton (™) in treatment of Chronic Gastroduodenitis of Eldery Patients. The 6th. International Medicinal Mushroom Conference. 2011, Zagreb, Croatia. P. 8-9.
132. Прилуцька А.Б. Порівняльна оцінка методів лікування гнійних ран в акушерській практиці: автореф. дис. …кандидата мед. Наук.- Київ, 2003. – 24с.
(Prylutska A.B. Comparative assessment of purulent wound treatment methods in obstetric practice: autoref. thesis ...professor of medical sciences. - Kyiv, 2003. - 24 p.)
133. Prilutskaya A.B., Prilutsky A.I.,Venckovsky L.B. et al. Cytological study of wound healing process with the use of chitin-containing preparation Mycoton /Advances in chitin sciences. Ed. by H. Struszczyk, A. Domard, M. G. Peter., H. Pospieszny – Poznan. 2005. -Vol.VIII.-P.236 –239
134. Senyuk, O.F. (1999). Research of Mycoton and Vivaton bio preparations as adaptogens to increased radiation exposure. Problems of Chernobyl,
Interdisciplinary Scientific and Technical Center of the National Academy of Sciences of Ukraine, Chernobyl (Ukraine).
